DYNASAN 118 is a triglyceride of selected saturated single fatty acid C18 of plant origin and plant derived glycerol.
DYNASAN 118
Supplier: IOI Oleochemicals
Product Applications: Hot Melt Extrusion, Ovule, Pharma Excipients, Suppository, Tablet, Topical drugs,
Product Class: Excipient, Solid triglycerides,
Product Category: Pharmaceutical
DYNASAN 118
Product Information
DYNASAN 118 is a triglyceride of selected saturated single fatty acid C18 of plant origin and plant derived glycerol. When DYNASAN 118 is rapidly cooled after melting, a glassy, amorphous mass is initially formed. Upon standing, it changes into crystalline modifications having volume expansion. The stable ß-modification has a very sharp melting point and a triclinic structure.
Melting Point [C°] ~72
DYNASAN 118 Applications
DYNASAN 118 is used in the pharmaceutical industries in the following application fields: – in tablets as a lubricant showing a very low influence on disintegration or as retarding agent if used in higher concentrations (2-5%).
- In suppositories, ovules and pharmaceutical sticks as crystallisation accelerators to improve congealing processes
- In ointments, creams and lotions for body imparting and structure forming effects
- In hot melt coating processes (taste masking)
- In hot melt extrusion processes (bioavailability enhancement)
- In solid lipid nanoparticles (SLN) procedures
DYNASAN – Versatile Excipients for Solid Dosage Forms
The DYNASAN® product range is a family of high-purity monoacid triglycerides. Their chemical nature, raw materials, manufacturing and purification result in products with advantageous properties and impurity profile.
The optimized degree of esterification leads to almost no free OH functionality on the backbone of the excipients and removes formulation and stability challenges in tablets, typically encountered with lubricants based on metallic salts of fatty acids*:
- Metal salt impurities interacting with APIs
- API interaction with lubricants due to their ionic characteristics
- Microenvironmental pH shift into alkaline milieu, creating a risk of drug hydrolysis
- Initiation of redox cascades degrading APIs – Metal ion-mediated degradation of APIs
- Ionic excipients and salt counter-ions show reactivity towards amines, which are common functional groups in APIs with metal soaps
Incompatibilities with metallic salts of fatty acids have been reported, e.g. for the following active ingredients: Acetylsalicylic Acid, Captopril, Besylate, Cephalexin, Clopidogrel Besylate, Erythromycin, Glibenclamide, Glimepiride, Ibuprofen, Indomethacin, Ketoprofen, Norfloxacin, Oxacillin, Penicillin G, Temazepam
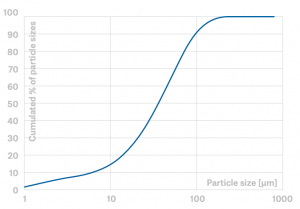
Particle Size Distribution of DYNASAN 118 (microfine powder)
Benefits for Tableting
- Effective lubricant for tablet production in low concentrations (~ 0.5%) without the risks of fatty acid soaps
- No influence on drug release up to a concentration of 1%, model drug Acetylsalicylic Acid
- Higher concentrations from 2 to 5% increase the hydrophobic characteristics of tablet matrix and introduce retardation effects into oral dosage forms for sustained release characteristics
- Enhances the tablet fracture stability due to an increased lubricity factor resulting from particle morphology and particle surface






